Pushpa Telugu Movie Review, Rating
పుష్ప తెలుగు సినిమా రివ్యూ ,రేటింగ్
-
One Movie She Promoted, One She Erased: Pooja Hegde’s Calculated Exit
-
“If You Don’t Click This, You Might Be Dead” — Why Millions Are Downloading This App
-
This Is NOT Visa-Free Germany — Germany Scraps a Painful Rule for Indians
-
Understanding the Impact of Northeast Toilet Placement According to Vastu Shastra
-
How to Open a Demat Account in India: Benefits & Tips
-
6 Effective Vastu Tips to Enhance Positive Energy in Your Home
-
Edge AI Consumer Devices & Trends in 2026🚀
-
Quick and Healthy Spinach Mushroom Spaghetti Recipe
-
Foods to Avoid for Healthy Hair: Combat Hair Loss Naturally
-
Delicious Almond Halwa Recipe for Winter Delights
-
DIY Body Lotion Recipe for Winter Skin Care
-
Essential Skincare Tips for Youthful Skin: Avoid Common Mistakes
-
The Health Risks of Drinking Water While Standing
-
Unhealthy Tea Pairings: What to Avoid for Better Health
-
Rising Lung Cancer Cases in India: A Growing Concern
-
Delicious and Nutritious Beetroot Paneer Dosa Recipe
-
Delicious Homemade Carrot Pickle Recipe for Winter
-
The Significance of Avoiding Hair Washing on Thursdays
-
Daily Milk Consumption: How Much is Ideal for Your Health?
-
The Risks of Drinking Water from Bottles Left in Hot Cars
-
Choosing the Perfect Colors for Your Kitchen Walls
-
Essential Tips for Safe Bottle Feeding Your Baby
-
10 Home Decor Mistakes to Avoid for a Stylish Living Space
-
Delicious Indian Dishes for Effective Weight Loss
-
Revitalize Your Skin with a Homemade Tomato Face Pack
-
Natural Drinks to Enhance Your Skin's Radiance
-
Astrological Insights: Foot Habits That Impact Your Life
-
The Benefits of Yogurt and Turmeric for Radiant Skin
-
Astrology Insights: The Fascinating Significance of Moles on Your Body
-
Essential Guide to Performing Tulsi Puja Correctly
-
Delicious Variations of Green Tea for Health Enthusiasts
-
Delicious Homemade Tomato Soup Recipe for Winter Warmth
-
Transform Leftover Rice into Delicious Rice Cutlets
-
Essential Tips for Choosing the Perfect Comb for Your Hair
-
Effective Pen Exercises to Enhance Children's Handwriting
-
Natural Remedies for Alleviating Menstrual Pain
-
Understanding Sciatica: Symptoms and When to Seek Help
-
Revitalize Your Hair with Coconut Oil: 5 Powerful Combinations for Healthy Locks
-
Post-C-Section Grooming: When It’s Safe to Wax and Tips for Smooth Recovery
-
Honda 2026 CB750 Hornet: 5 Reasons Its e-Clutch Tech Will Blow Your Mind
-
Mahindra XUV 7XO: 5 Key Features It Surprisingly Misses Despite Advanced Tech
-
Tesla Is Coming to Bengaluru: 5 Things to Know About the EV Giant’s India Debut
-
Understanding the Pros and Cons of Coconut Oil for Hair Care
-
KTM RC 160: Top 5 Defining Features That Make It a Street Beast
-
Suzuki Motorcycle India News – 10 Million Production Milestone
-
🏆 Overview: Sustainable Weight Loss ≠ Quick Fix
-
Overview of the CMAT 2026 selection process
-
🌞 What Is Daan (Charity) on Makar Sankranti?
-
“Social Jetlag: Symptoms, Causes, Side Effects, and Tips to Reset”
-
The Hidden Dangers of Stalking: Impact on Women's Heart Health
-
FSSAI Food Analyst Examination 2025
-
Ather Energy announcement that opened its fast charging network to non Ather electric vehicles (EVs)
-
SCR Special Trains for Sankranti Travel Rush
-
Eggless Butter Cake Recipe
-
MG Majestor — Fortuner Rival SUV (Jan 2026)
-
📧 Why Are People Getting Instagram Password Reset Emails?
-
Chocolate Biscuit Cake Recipe (No-Bake)
-
🚆 Bihar Gets Direct Link to Southern States as Railways Launches New Trains
-
🚆 Amrit Bharat Express Launched: Three New Trains to Connect Tamil Nadu and West Bengal
-
ISRO’s First 2026 Launch — “Eye in the Sky” satellite📡🇮🇳
-
🚆 New Jalpaiguri–Tiruchirappalli Amrit Bharat Express: Key Stops & Route
-
Sour Cream Coffee Cake Recipe
-
Suzuki e Access launch in India — covering price, warranty, specs, features, offers and what riders need to know:
-
Sealdah Banaras Amrit Bharat Express: Check Route, Timetable and 6 Key Stops
-
Punjab strengthens maternal care through Aam Aadmi Clinics
-
Mango Muffins Recipe (Eggless)
-
📉 Big iPhone 15 Discounts: What’s Happening Now
-
📱Realme P4x Overview: A Budget Powerhouse with a Massive Battery
-
📱 Jio’s New 36 Day Recharge Plan — What It Is
-
💡 Why the IT Department Tracks Transactions
-
“From Chandrayaan 1 to PSLV C62: The evolution of ISRO’s perfect satellite launch vehicle”,
-
🔍 Why You Got a Password Reset Email from Instagram
-
Eggless Carrot Cake Recipe (Whole Wheat)
-
📢 PhonePe Launches PG Bolt to Enable One Click Card Payments with Visa and Mastercard
-
🧹 Dreame X40 Ultra Review: Luxury Meets Precision in Home Cleaning
-
🎉 Jio Introduces ₹450 Festive Offer With 2GB Daily Data & OTT Subscriptions
-
🔔 Reliance to Double Investment in Gujarat to ₹7 Lakh Crore
-
Jio to Launch a People First AI Platform ‘Built in India’ for India and the World: Mukesh Ambani
-
Got an Instagram Password Reset Email Without Asking? What It Means and How to Stay Safe
-
Eggless Blueberry Muffins (Whole Wheat)
-
📱 Source Code Talks Are “Routine,” Not a New Smartphone Security Alarm
-
Why Your Body Holds Stress Even After Work Hours
-
Regional AI Impact Conference 2026 in Lucknow
-
🎉 Jio Update: Enjoy YouTube Premium for Free — No Extra Fees
-
Are You Losing Money Through Unauthorized UPI Transactions? How to Stop Fraud Before It Happens
-
You Could Get an Electric Shock if You Don’t Check for This Coating on Water
-
Honey Cake Recipe (Bakery Style)
-
Bank Statement Explained: Why Your Account History Reflects Your True Financial Health
-
Credit Card Closure Risks: How Cancelling a Card Can Increase Credit Risks
-
8th Pay Commission & DA News Highlights
-
SIP and SWP Strategy: Securing Your Long-Term Financial Goals with Mutual Funds
-
Rava Cake | Suji ka Cake Recipe
-
🩺 Health Insurance Alert: Small Mistakes Can Lead to Big Losses During Claims
-
📊 Personal Finance Insight: High Income Doesn’t Always Mean Bigger Loans
-
📞 UP Board Exam 2026: Official Helpline & Support Contacts
-
Strawberry Cake Recipe (With Cream Icing)
-
How to Delete Your ChatGPT Account: Step-by-Step Guide
-
🧾 Senior Citizen FD Investment: How to Avoid TDS on Fixed Deposit Interest🧾 Senior Citizen FD Investment: How to Avoid TDS on Fixed Deposit Interest
-
Zoho Arattai’s 10 Powerful Features That Could Make You Quit WhatsApp Today
-
CBSE Counseling 2026: IVRS & Tele Counseling Services for Board Students
-
🔔 Government Jobs Alert 2026: Top 6 Recruitments with ~3,231 Vacancies
-
How to Enable 2-Step Verification on Your Google Account: A Complete Safety Guide
-
Cucumber Cake Recipe | Dhondas | Tavsali
-
How to Check If Your Google, Facebook, or Twitter Passwords Are Compromised
-
How to Use Text-to-Speech and Voice Effects on Instagram Reels: Step-by-Step
-
How to Use Google Photos Locked Folder to Hide Private Photos and Videos
-
How to Add a Customized Link Sticker to Your Instagram Story: Step-by-Step
-
How to Identify a Fake PAN Card Using QR Code: A Simple Verification Guide
-
This Is Why Congress Loses Always - Confusing Commercial Cinema for Cultural Identity
-
WhatsApp Calls Not Ringing When Phone Is Locked? Here’s How to Fix It
-
New Income Tax Act from April 1: 64 Year Old Law Ends, Filing Taxes to Change
-
Eggless Pineapple Cake Recipe | Pineapple Pastry
-
WhatsApp Voice Message Preview Feature: How to Listen Before Sending
-
8th Pay Commission Update: Central Employees May Receive Up to 5% DA Increase
-
Post Office Savings Schemes 2026: Latest Interest Rates and Best Low Risk Options
-
PM Kisan Yojana Update: Installment Will Stop Without e KYC — Know the Simple Process
-
Punishment for Not Returning Money Mistakenly Deposited in Your Account
-
Classic New York Cheesecake (Eggless)
-
UPSC Exam 2026: Face Recognition Now Mandatory at Exam Centres — New Rules Explained
-
How to Become an Officer in the Indian Army: Exams and Requirements
-
Alert for Bank Customers: Banks to Remain Closed in These Cities
-
Now You Can Add a Cover Photo to Your WhatsApp Profile! This New Feature Is
-
Why Does a Loan Get Stuck Even With a Good Income and Credit Score?
-
No-Bake Mango Cheesecake (Eggless)
-
Smartphone Industry News: Apple & Samsung in 2026
-
Did You Also Receive an Email to Change Your Instagram Password? Here’s What It Means
-
Budget Demand: Don’t Increase Income Tax Surcharge on the Super Rich
-
BTech vs BSc: Which Course is Better for You? Career Scope Explained
-
Indian Students at High Risk Amid Australia Visa Fraud Crackdown
-
Sunil Grover Kicks Out Aamir Khan? Wildest Marketing Stunt for ‘Happy Patel’
-
Crushing Rejection: Travel History Flags B-1 Visa Applicant
-
Actress Priyanka Chopra Stuns at the Golden Globes
-
‘Mana Shankara Vara Prasad’ First Half: Entertaining and Engaging
-
Murari’s ‘Safe Game’ in the Packed Sankranti Release Race
-
“Language Is Not a Barrier; Story Is What Matters” – Kalyani Priyadarshan
-
Who Will Lead Anil Ravipudi’s Landmark Film?
-
‘Sarvam Maya’ Dominates Theaters; Fans Eager to Know OTT Release Date
-
Ranveer Singh’s ‘Dhurandhar’ Shakes Box Office, Earns ₹120 Crore Worldwide in First Month
-
Chennai High Court Imposes Interim Ban on Commercial Use of Kamal Haasan’s Name and Image
-
Second Single from ‘Jackie’ Released; Film Centers on Traditional Madurai Sport
-
83rd Golden Globe Awards Announced: Celebrating Global Cinema in the U.S.
-
Atharvaa Shares Behind-the-Scenes Photos from ‘Parasakthi’; Film Earns ₹27 Crore on Day One
-
Karthi’s ‘Vaa Vaathiyar’ to Release on January 14: Crew Official Announcement
-
Prabhas’ ‘Raja Saab’ Smashes Box Office, Earns ₹183 Crore in Just 3 Days
-
Pawan Kalyan Impresses in Martial Arts; Receives International Recognition
-
“Music for Meals”: Fundraising Concert Receives Praise for Social Service; Ilaiyaraaja Lauded
-
Pongal Releases: Karthi’s Film Gets Green Signal; Jeeva Excited, Santhanam’s Film Delayed?
-
Napoleon to Produce and Act in Film in the United States
-
Lyricist Priyan Turns Hero in Upcoming Films
-
Vadivukarasi to Play Lead Role in Story-Driven Film
-
Nagesh Peran to Star in New Film ‘Manasum Manasum’
-
Commercial Use of Kamal Haasan’s Name and Image Prohibited
-
Ajith’s 64th Film to Be Announced During Pongal
-
“Attakathi” Dinesh Skips Pongal Celebrations Amid Busy Shoot for ‘Karuppu Pulsar’
-
Who Will Star Next in Sudha Kongara’s Direction — Simbu or Dhruv Vikram?
-
Meenakshi Chaudhary Found Comedy Roles Challenging
-
When Will the Saidapet–Teynampet Flyover Be Opened? Health Minister Ma. Subramanian Makes Key Announcement
-
Road in Erode Named ‘Thiyagi Kumaran Road’; Chief Minister M.K. Stalin Pays Tribute
-
How India Lowered the Bar for Humour — The Tanmay Bhatt Problem
-
Where the World Is Having the Most Sèx — India’s Ranking Will Shock You
-
Bhartha Mahasayulaku Wignyapthi Review - Ravi Teja delivers, the writing hesitates
-
UNINSTALLED YET EXPOSED: The Shocking Truth About Why Your Name Still Shows Up on Truecaller
-
Sharper Than Reality: The AI Images That Hijacked Pooja Hegde’s Viral Moment
-
Double-Decker Bus Service Reintroduced in Chennai; Flagged Off by Chief Minister M.K. Stalin
-
“We Are All Tamil Kin Who Cannot Be Divided by Anyone,” Says Chief Minister M.K. Stalin
-
Union Government Not Providing Funds for the Development of Classical Languages, Says MP Kanimozhi
-
Sanitation Worker Padma Rewarded with ₹1 Lakh by Chief Minister Stalin for Honesty in Returning 45 Sovereigns of Gold
-
Sweet News for Women: Women’s Rights Assistance Likely to Be Increased by 50 Percent
-
Minister Sekarbabu and Mayor R. Priya Distribute Samathuva Pongal Gift Hampers to 550 Beneficiaries
-
Congress Found Its Voice — Sadly, Only at the Cinema Hall
-
The Superman Comic That Just Sold for ₹135 Crores : They Stole It, Lost It, Found It—and Sold It for ₹135 Crores
-
No Officials. Just a Bellboy - India's Union Minister Received by Bellboy in USA
-
One of the Fastest Renewals in Streaming History? Fallout Season 3 Is Already Powering Up
-
Is Vijay the BJP’s Masterstroke Against DMK in 2026? Behind Closed Doors in Delhi - Vote Split Is the New Weapon
-
January 12 ,2026 Daily Panchagam
-
Eggless Gingerbread Cake Recipe | Gingerbread Loaf
-
Which cricketer was born on 12 January?
-
January 12,2026 Daily Horoscope(Astrology)
-
Whose birthday is on 12 January in India?
-
What is celebrated on 12 January in India?
-
Wine Cake Recipe (Eggless) Explain
-
What is the 12 January known as?
-
What is the World day on 12 January?
-
What national food day is 12 January?
-
What is the festival on 12 January?
-
What is the personality of someone born on 12 January?
-
Applesauce Cake Recipe (Eggless)
-
What is special about the 12 January?
-
What happened on 12 January in history?
-
Not One or Two, But 13 Students Land Job Offers from Google; Truly Spectacular
-
Will Anil Ravipudi Continue to End His Heroes’ Flop Streak?
-
Mumbai's Digital Political War: The Opposition Fails to Keep Up with the BJP's "Marvel-Style" Campaign
-
Mumbai's Development Under Devendra Fadnavis: Ten Years of Growth, Infrastructure, and Connectivity
-
Greenland 2: Migration Review – Surviving The End Was Easy. Living After It Is Hell
-
Primate Movie Review – Let the Chimp Cook — and Kill
-
Golden Globes 2026 - Check The Complete Winners List
-
How ‘Parasakthi’ Became a Target of Cyber Hate - Paid Negativity, Planned Outrage
-
2014 vs 2025: The Adani Explosion That Lit Up X—and India
-
If Time Was Managed in Delhi, Why Was Karur Left to Fate? A Question Vijay Cannot Dodge
-
Ram Pothineni’s 20-Year Journey Wins Hearts Across Audiences
Empowering 140+ Indians within and abroad with entertainment, infotainment, credible, independent, issue based journalism oriented latest updates on politics, movies.
India Herald Group of Publishers P LIMITED is MediaTech division of prestigious Kotii Group of Technological Ventures R&D P LIMITED, Which is core purposed to be empowering 760+ crore people across 230+ countries of this wonderful world.
India Herald Group of Publishers P LIMITED is New Generation Online Media Group, which brings wealthy knowledge of information from PRINT media and Candid yet Fluid presentation from electronic media together into digital media space for our users.
With the help of dedicated journalists team of about 450+ years experience; India Herald Group of Publishers Private LIMITED is the first and only true digital online publishing media groups to have such a dedicated team. Dream of empowering over 1300 million Indians across the world to stay connected with their mother land [from Web, Phone, Tablet and other Smart devices] multiplies India Herald Group of Publishers Private LIMITED team energy to bring the best into all our media initiatives such as https://www.indiaherald.com

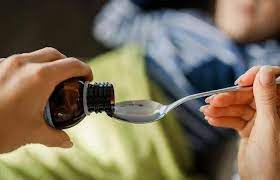 In this regard, in a letter sent by the Drug Control Authority, a package of products made using a cocktail of two drugs, chlorpheniramine maleate and phenylephrine, has been requested. These medicines help relieve cold and flu symptoms, including watery eyes, runny nose, sneezing, and itchy nose or throat. While chlorpheniramine maleate acts as an anti-allergic agent, phenylephrine acts as a decongestant, constricting small blood vessels to provide relief from nasal congestion or congestion.
In this regard, in a letter sent by the Drug Control Authority, a package of products made using a cocktail of two drugs, chlorpheniramine maleate and phenylephrine, has been requested. These medicines help relieve cold and flu symptoms, including watery eyes, runny nose, sneezing, and itchy nose or throat. While chlorpheniramine maleate acts as an anti-allergic agent, phenylephrine acts as a decongestant, constricting small blood vessels to provide relief from nasal congestion or congestion. However, later, various concerns have been raised against the use of these drugs among infants and children, it said. This was discussed in the Expert Panel (Lung) on june 6. The committee recommended that these drugs should not be used in children below 4 years of age and accordingly, companies should mention a warning to this effect on the label and package.”
However, later, various concerns have been raised against the use of these drugs among infants and children, it said. This was discussed in the Expert Panel (Lung) on june 6. The committee recommended that these drugs should not be used in children below 4 years of age and accordingly, companies should mention a warning to this effect on the label and package.” 



 click and follow Indiaherald WhatsApp channel
click and follow Indiaherald WhatsApp channel