Pushpa Telugu Movie Review, Rating
పుష్ప తెలుగు సినిమా రివ్యూ ,రేటింగ్
-
Kiara Advani Drops One Photo. The Internet Loses Its Mind.
-
Millie Bobby Brown Just Broke the Internet — Stranger Things 5 Is Silent But Millie Is Not
-
Who Is Bandana Girl? How a 2-Second Clip Broke the Internet and What She Did The Amount She Earned On X?
-
Pooja Hegde Just Froze Instagram — And Melted Every Heart in the Process
-
AVENGERS: DOOMSDAY Trailer Incoming — Marvel Is About to Drop Its Biggest Trailer Since ENDGAME
-
Daisy Ridley Backs The Fan Movement Trying to Revive a Film Disney Never Wanted You to Know About.
-
Séx-for-Rent Exploitation Explodes — And These Countries Are Failing Their Most Vulnerable
-
Thoothukudi: The District Everyone Forgets — Until They Need Salt on Their Plate.
-
McDonald’s, KFC, Pizza Hut to Take Over Stations — But Who’s Fixing the Tracks?
-
India’s Dark Habit: Fixing Problems Only After Bodies Fall.
-
Under 16? No X. No Facebook. No Insta. No Scroll. Australia Drops the Mother of All Bans.
-
Vijay Praised the Govt That Just Shrunk His Rally — Is Vijay Really Aware Of What's Happening Around Him?
-
The Rupee Is Sinking — And the RBI Is Running a Secret, High-Stakes Operation to Keep It From Drowning.
-
Kashi’s New Cricket Stadium Uses Trishul Lights — Netizens Respond With Divine Levels of Sarcasm.
-
China Hangs Corrupt Officials — But in India, They Get BJP's Magic Wash
-
25 Years Later Padayappa STILL Eats, Leaves No Crumbs, and Walks Away.
-
The Plot Twists. The Bloodlines. The Nightmares. The Romances. THESE Were the 10 Shows That Owned 2025.
-
India in 'Reform Express' Phase, Says PM Modi
-
Understanding DGCA, Airport Authority of India, and BCAS: Roles, Functions, and Differences
-
New Labour Code: How It Affects Your Take-Home Salary & PF Deductions – Complete Rules Explained
-
Oriental Insurance Company Limited Recruitment 2025: Apply Soon for Administrative Officer Positions
-
8th Pay Commission: Will Salaries Increase from Next Month? Government Clarifies
-
Bihar Government Launches Civil Services Incentive Scheme for Female Candidates: ₹50,000 Incentive Available
-
Indian Railways RRB JE Recruitment 2025: Last Chance to Apply for 2,600 Junior Engineer Posts
-
Hotel Booking Scams on the Rise: How Scammers Trap People Online
-
Uttar Pradesh Government’s Kanya Sumangala Yojana: A Financial Lifeline for Daughters from Birth to Schooling
-
How to Obtain Passes for the 26th January Parade: The Easiest Way to Book Tickets
-
Nivetha Pethuraj Deletes Her Engagement Pics Overnight — Breakup ALERT !!?!
-
Rashmika's ‘Thamma’ Finally Streams for Free — Here’s When to Hit Play!
-
RTI BOMBSHELL: Did BJP Raise Party Funds by Masquerading as Government Schemes?
-
Sky-High Privilege for Politicians, Grounded Misery for People — Who Really Pays the Price?
-
South Africa Folded Like Wet Cardboard: Hardik Pandya’s 59(28) Sparks India’s 101-Run Demolition
-
₹15 Crore Evaporated in 10 Hours: Did Modi Just Turn Parliament Into a Political Stage?
-
Shabana Azmi Celebrates 41st Wedding Anniversary with Javed Akhtar
-
Kartik Aaryan Reveals Initial Rejection of Bhool Bhulaiyaa Sequel
-
Unsold in IPL 2025 Mega Auction, Prithvi Shaw Sets Base Price for IPL 2026 Auction
-
Travel Time Between Kanpur, Delhi, and Mumbai to Reduce with New 112 km Highway in UP
-
Who Is Neal Mohan? TIME Names YouTube CEO ‘CEO of the Year 2025’
-
"Don't Overexpose Varun!" THIS World Cup Winner Warns India Ahead of T20 World Cup 2026
-
From Quinoa to Flaxseeds: 10 Superfoods to Naturally Manage High Uric Acid Levels
-
'Kaantha' OTT Release Date Announced: When and Where to Watch Dulquer Salmaan's Hit Tamil Movie
-
Virat Kohli to Finally Appear on 'The Great Indian Kapil Show'? Kapil Sharma Makes Big Revelation
-
Ranveer Singh’s ‘Dhurandhar’ Secures OTT Deal Amid Box Office Success
-
Preparing for NIFT Admission 2026? Registration Opens; Exam on February 8
-
7 Yoga Asanas That Can Provide Instant Relief from Gas
-
Is Drinking Pudina (Mint) Water After Lunch Effective for Indigestion?
-
China Tests ADAS-Type Technology to Wirelessly Link Seven Freight Trains
-
8 Breakfast Options for a Healthy Gut: Doctor Shares List
-
CSIR UGC NET Exam City Intimation Released Ahead of December 2025 Exam
-
World’s Top 10 Business Schools for MBA: A Global Perspective
-
Is Your Room Heater Harming Your Health? Doctor Shares Safe Winter Tips
-
WBCSSC Extends Application Deadline for 8,477 Group C and D Vacancies
-
Why Delhi University's Online MBA Is Gaining Popularity: Fees, Eligibility, and Specialisations
-
Staying Indoors in Winter Can Increase Risk of Colds: Try These Ventilation Tips
-
IIT Madras Launches Advanced Certificate Programs in Artificial Intelligence
-
Dreaming of a UN Career? Apply for the Young Professionals Programme by December 14
-
Manipur COHSEM Announces 2026 Class 10 and 12 Board Exam Dates
-
4 Natural Remedies for Winter Hair Fall and Dandruff
-
High Cholesterol? Make These Diet and Lifestyle Changes Immediately
-
CBSE Class 12 Exams 2026: Key Competency-Focused Chemistry Questions to Practice
-
Delhi University Extends Scholarship Application Deadline for Academic Year 2025-26
-
Speed Over Safety? 10-Minute Medicine Deliveries by Blinkit and Zepto Raise Concerns
-
Is Poor Sleep Discipline Causing Forgetfulness in Young Professionals? Doctor Says Yes
-
How to Score High Marks in CBSE Class 10 Mathematics: A Complete Guide
-
Banaras Hindu University Invites Applications for MBA Programmes – Check Details to Apply
-
20 Most Googled Symptoms in India This Year and Their Possible Causes
-
NTA Releases SWAYAM Admit Card 2025 for July Semester
-
Comic Review: Chintu & Bantu – A Fun and Hilarious Take on the ‘EPIC’ Glimpse
-
OTT Review: Aan Paavam Pollathathu – Telugu-Dubbed Tamil Film Now Streaming on Jio Hotstar
-
Review: Ranveer Singh’s Dhurandhar – A Gripping Spy Action Thriller
-
Champion: Roshan Meka and Anaswara Rajan Shine in the ‘Sallangundaale’ Song
-
Disadvantages of Eating Jaggery in Excess
-
Rs 130 Crore OTT Deal: Netflix Acquires Ranveer Singh’s Spy Thriller Dhurandhar
-
Hyderabad to Get Artificial Beach and Tunnel Aquarium: A New Urban Attraction
-
100 Days to Go: Dead Air Everywhere! – Yash’s KGF Momentum Continues
-
Hangover Remedies: Kitchen Ingredients to Know Before Holiday Parties ..
-
Tamannaah’s Big Shift – From Glamour Roles to Biopic
-
Akhanda 2 Gets Court Approval, New Release Date Locked!
-
Yash’s Toxic: 100-Day Countdown Poster Takes Fans by Storm
-
Chief Minister Lays Foundation Stone for Construction of Mini Stadiums Across Tamil Nadu
-
DMK Will Form Government Again in Tamil Nadu: Announcement at High-Level Party Meeting
-
DMK’s “My Booth, Victory Booth” Campaign to Begin Tomorrow: Chief Minister M. K. Stalin to Launch the Programme
-
Grand Thirukalyana Utsavam Celebrated at Kanchipuram Ekambaranathar Temple: Thousands of Devotees Take Part
-
Bhavani Municipal Secretary Nagarajan Passes Away: Chief Minister M. K. Stalin Expresses Condolences
-
Male Infertility: Increasing Trends and Causes
-
Ban on Digital Promotion of Antibiotics & Prescription Drugs: Risks to Public Health
-
Rahul Gandhi Slams RSS Over ‘Institution Capture’
-
PM Modi Urges Citizen-First Governance at NDA Meet
-
“Samsung Galaxy S26, S26 Plus, S26 Ultra Leaks: Launch Date, Price, Specifications, Design, and More”
-
“The Last Pradosh Vrat of 2025: Date, Timings, and How to Observe It”
-
“Why Egg Yolks Were Never a Problem for Your Heart – Doctor Calls Out ‘Big Scam’”
-
“From Party to Prevention: Why Even Young Adults Should Watch Their Hearts in 2026”
-
“Blindness, Stroke Among Hidden Risks of Cosmetic Fillers, Researchers Warn”
-
“From Dementia to Cancer: What Body Odours Reveal About Your Health”
-
“Constipation Is the New Office Epidemic – And Your AC Might Be to Blame”
-
“Indian Motorcycle Challenger Venum Cruiser to Make Global Debut on December 13”
-
“2026 Mercedes-Benz GLB Electric 7-Seater Launched – Battery, Range, Features”
-
“New MG Hector SUV Facelift Teased – Here’s What to Expect”
-
“Royal Enfield Meteor 350: Price, Colours, Features, Engine, Mileage, and More”
-
“ICAI CA Jan 2026 Admit Card Expected Soon: Exam Dates and Download Steps”
-
“MCC NEET PG Round 2 Counselling 2025: 2620 Seats Added for MD, MS, and DNB Programs”
-
“Lucknow University Admit Card 2025 Released: Step-by-Step Download Guide”
-
“Australia’s UNSW Sydney Gets UGC Approval to Open Campus in Bengaluru; Classes From August 2026”
-
“ITR Forms Under New Income Tax Act to Be Notified Before FY28: Government Announcement”
-
“OnePlus Pad Go 2 First Impressions: A Big Style Upgrade”
-
“SNAP Test 2 Admit Card 2025 Released: How to Download and Key Details”
-
“Gmail New Feature: Say Goodbye to Unwanted Emails in Your Inbox”
-
“Year-End 2025: WhatsApp Gets Exciting New Features to Make Chatting More Fun”
-
“Why Your ITR Refund Hasn’t Arrived: Shocking Reasons Behind the Delay”
-
Kerala CM Rejects Dileep’s Conspiracy Claim
-
“How to Set Your Favorite Song as a Caller Tune on BSNL”
-
“UPSC CSE 2025 Interview: Admit Cards Released for Personality Test”
-
“Beware of e-PAN Card Email Scams: Don’t Click the Link!”
-
“Small Investments, Big Returns: Top 5 Investment Options for Beginners to Build a Strong Future”
-
Amit Shah Blames Nehru–Indira for Partition; Kharge Retaliates
-
“What a Nominee Can Claim After the Death of a Working Member: It’s Not Just PF”
-
“Jan Dhan Accounts Cross ₹2.74 Lakh Crore: Half of India Now Has a Bank Account”
-
“How to Properly Close a Settled Loan: Avoid Mistakes That Can Affect Your Credit for 7 Years”
-
“RBI Cuts Repo Rate, But Your EMI Hasn’t Decreased? Here’s How to Lower Your Loan Interest Immediately”
-
OTT deal value for Dhurandhar ..?
-
Saumya Tandon Praises Akshaye Khanna ..!
-
Vijay Varma Opens Up on Public Relationship After Breakup ..?
-
Hema Malini Holds Another Prayer Meet for Dharmendra; Daughters Esha and Ahana to Attend
-
Aamir Khan Returns: Sequel to ₹400 Cr Film Confirmed ..
-
Priyanka Chopra Melts at Malti’s Adorable Gift ..!
-
Box Office: Ranveer Singh Outshines ‘Sikander’
-
At 33, Rhea Chakraborty Freezes Her Eggs — Shares Insights on Future Plans
-
Farah Khan Praises Akshaye Khanna After Watching Dhurandhar ..?
-
Tanya Mittal on the "Fake" Tag ..?!
-
Vijay Proves Why He's a 'Tharkuri' Again In Puducherry - Reading Wrong ‘Fact’ Boldly On Stage
-
Shriya Saran’s Sun-Drenched Bikini Diary From Wayanad Is Pure Editorial Gold
-
Metallic. Sculpted. Unapologetic. Manushi Just Redefined Red-Carpet Power
-
From Breasts-Covering Caution to Glam Dance With Salman - Keerthy Suresh 's Double Standards Exposed
-
Netflix vs. Paramount: Hollywood’s $80 Billion Knife Fight Just Turned Into a Corporate Bloodbath
-
“Don’t Tell Me My Job!” — Puducherry Lady Cop’s Explosive Warning to Bussy Anand Shakes Vijay’s Meeting
-
Telugu Actress Teacher Boyfriend Gave Her Papers And A+ Favors – “Nothing Physical” She Swears
-
Bengaluru Temple Bans Weddings — ‘More Divorces Than Blessings’
-
15 Countries That Can’t Stop Watching Porn — And the Internet Knows Everything
-
Goa Fire, Delhi Airport Exit, IndiGo Escape — And Still No Questions for the BJP Government?
-
Goa Burns, IndiGo Collapses, Air Turns Toxic — And Parliament Debates Vande Mataram for 10 Hours
-
The Rise, The Hype, The Collapse — Vijay’s Political Aura Just Imploded
-
Tourism Mafia Thrives, Tourists Die — No NOC. No Exits. No Safety. No Accountability As Goa Demands Answers.
-
Buffaloes Enter the Ground But TVK’s Virtual Soldiers Enter Only Comment Sections
-
From Mallya to the Goa Fire Case — How India’s Most Wanted Keep Clearing Immigration Under BJP Rule
-
₹11,820 Crore Gone in 7 Days: The Economy Slips, the Rupee Falls, and Modi Blames Nehru
-
Why Tamil Nadu Drivers Are Finally Breathing Again — Tamil Nadu’s Infrastructure Makeover Has Officially Begun
-
This Is Not Turbulence — Critics Call It the BJP Government’s Full-Blown Aviation Failure
-
India’s Telecom & Aviation Monopolies Are Back — And This Time, No A. Raja To Stop Them
-
“Make Your Ayushman Card from Home: Step-by-Step Easy Process”
-
“KVS-NVS Vacancy 2025: Application Deadline Extended for 14,900+ Posts”
-
“Constable Jobs 2025: Over 1,700 Vacancies Announced for 10th and 12th Pass Youth”
-
“Group D Vacancy 2025: Recruitment Announced for 10th Pass Candidates — Basic Salary Up to ₹56,000”
-
Railway Vacancy 2025: Apply Soon for 2,585 Posts – Salary Up to ₹44,900
-
How Marriage and Divorce Affect Your Income Tax in India: A Complete Breakdown
-
Career Tips: 5 Ways to Protect Your Job from Layoffs
-
FD Investment: Falling Interest Rates – Should You Distance Yourself from Fixed Deposits?
-
Credit Score & New Loans: Does Borrowing Hurt Your Score?
-
Auto Loan EMIs Set to Drop After RBI Rate Cut: How Much You Can Save on Car and Bike Loans
-
AI Toys Raise Alarm: Inappropriate Conversations Spark Parental Concerns
-
India Plans to Reduce Electricity Bills Using Artificial Intelligence: How It Will Work
-
“FBI: Why Sending WhatsApp Messages Is Safer Than SMS — Warning Relevant to Everyone”
-
Starlink Launches in India: Recharge Plans, Dedicated Website, and 30-Day Free Trial
-
“‘Stop Using Google Chrome Immediately’: Why Apple Issued This Warning and What They Are Afraid Of”
-
Starlink India Pricing Revealed: Monthly Plan Cost and Benefits Explained
-
“NTA Releases SWAYAM July 2025 Exam City Slip: How to Download Your Exam Details”
-
“RBI Floating Rate Bonds: Complete Guide to Interest, Tax Rules, Eligibility, and Key Benefits”
-
“8th Pay Commission Implementation Still Uncertain: Finance Ministry Clarifies Timeline in Parliament”
-
“Apple-Google Warning: Spyware Is Trying to Infiltrate Your Phone — Do These Important Things to Protect Yourself”
-
“Projector vs. TV: The Biggest Drawback of Projectors That Makes Them Fall Short Compared to TVs”
-
“Update Aadhaar Mobile Number from Home: UIDAI Launches New Feature in Aadhaar App”
-
“Uninstall These 5 Apps From Your Samsung Smartphone Immediately; They Are Hogging Memory and Draining the Battery”
-
“To Prevent Your Child’s Eyesight from Weakening While Watching TV, Change These Settings Immediately”
-
“Install This App on Your Sisters’ and Daughters’ Phones — Help Arrives at One Touch in Times of Trouble”
-
“ChatGPT: Never Share These 5 Things With ChatGPT or Gemini — It Could Bring a ‘Mountain of Troubles’”
-
Smartwatch: Should You Wear It While Sleeping or Not? Knowing the Facts Will Help You Make the Right Decision
-
Banking: Get Cheaper Loans Now! EMIs Will Reduce as These 4 Banks Cut Their Interest Rates
-
Following the RBI, This Government Bank Has Also Cut Interest Rates — Your Home Loan EMI Will Now Decrease
-
Personal Loan: Get Money in Just a Few Minutes — Learn About the 7 Major Benefits of Personal Loan Apps
-
Want to Come Alive After Your Death? Just Pay ₹1.74 Crores
-
THOOTHUKUDI IS ABOUT TO BECOME INDIA’S NEXT ECONOMIC NUCLEAR REACTOR
-
When Kids With Half-Grown Moustaches Become Fathers — 'Gutur Gu' Is Pure Brain Rot Disguised as Content
-
T20 WORLD CUP 2026 BLACKOUT? JIO HOTSTAR’S MASSIVE EXIT LEAVES CRICKET FANS STUNNED
Empowering 140+ Indians within and abroad with entertainment, infotainment, credible, independent, issue based journalism oriented latest updates on politics, movies.
India Herald Group of Publishers P LIMITED is MediaTech division of prestigious Kotii Group of Technological Ventures R&D P LIMITED, Which is core purposed to be empowering 760+ crore people across 230+ countries of this wonderful world.
India Herald Group of Publishers P LIMITED is New Generation Online Media Group, which brings wealthy knowledge of information from PRINT media and Candid yet Fluid presentation from electronic media together into digital media space for our users.
With the help of dedicated journalists team of about 450+ years experience; India Herald Group of Publishers Private LIMITED is the first and only true digital online publishing media groups to have such a dedicated team. Dream of empowering over 1300 million Indians across the world to stay connected with their mother land [from Web, Phone, Tablet and other Smart devices] multiplies India Herald Group of Publishers Private LIMITED team energy to bring the best into all our media initiatives such as https://www.indiaherald.com
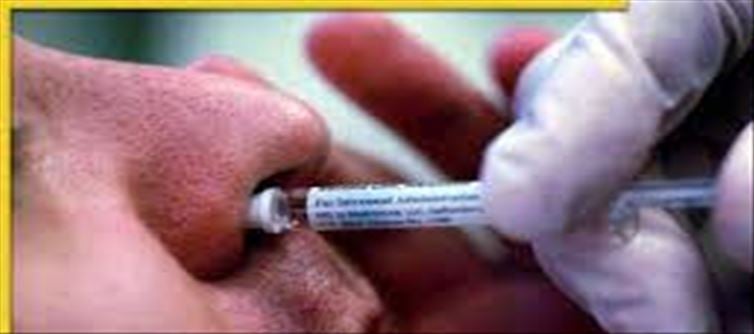
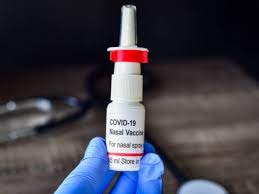 This is a huge boost in India's fight against the Corona epidemic! Bharat Biotech's ChAd36-SARS-CoV-S Covid-19 (chimpanzee adenovirus vector) recombinant nasal vaccine has been approved by the Central Medicines Quality Control System. Union health minister Mansukh Mandaviya said through a tweet that emergency use has been allowed for people above 18 years of age. In his next tweet, he said that this move further strengthens our collective efforts against the pandemic. He has said that india is making progress in the fight against Covid-19 under the leadership of prime minister Narendra Modi.
This is a huge boost in India's fight against the Corona epidemic! Bharat Biotech's ChAd36-SARS-CoV-S Covid-19 (chimpanzee adenovirus vector) recombinant nasal vaccine has been approved by the Central Medicines Quality Control System. Union health minister Mansukh Mandaviya said through a tweet that emergency use has been allowed for people above 18 years of age. In his next tweet, he said that this move further strengthens our collective efforts against the pandemic. He has said that india is making progress in the fight against Covid-19 under the leadership of prime minister Narendra Modi.



 click and follow Indiaherald WhatsApp channel
click and follow Indiaherald WhatsApp channel