First test kit authorized by ICMR to detect Omicron?
There is currently a delay in india to accurately diagnose this omicron infection which is spreading at an astounding speed. The Medical Research Council of india has approved Tata's OmiSure test kit, a diagnostic variant of the Omicron variant corona. The nations of the world have been facing the onslaught of the coronavirus for two years, 2020 and 2021. The threat of corona continues in the new year 2022 with a new variant called Omicron.
There is currently a delay in india to accurately diagnose this omicron infection which is spreading at an astounding speed. This is because Omicron cannot be detected by experimental methods, including RTPCR, which detect other corona variants. The omicron test is currently being performed in india using a test kit made by Thermo Fisher, an American scientific instrument company. Omicron infection is diagnosed by the S Gene Target Failure (SGTF) test method. This test is performed by targeting genes such as 'S' Gene, ORF, 'N' gene, Rdrp, 'E' gene in the coronavirus.
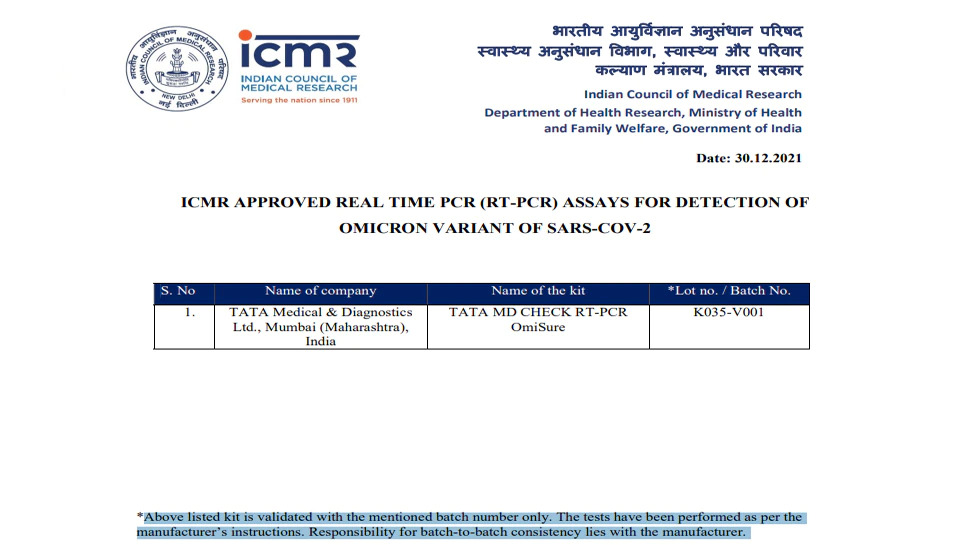
Meanwhile, the Medical Research Council of india (ECI) has approved the OmiSure, a unique OMICR diagnostic test kit developed by Tata Medical and Diagnostic Company, a mumbai - based company. The approval for this was given on december 30 last year. Tata's new testing kit is expected to be able to quickly diagnose omicron infection with ICMR approval. It is expected to add strength to the healthcare industry's action against omicron.




 click and follow Indiaherald WhatsApp channel
click and follow Indiaherald WhatsApp channel