🔴 FAKE ORS BAN: FSSAI SLAMS THE BRAKES ON MISLEADING 'HEALTH' DRINKS
Eight Years, Countless Lies, and a Late But Bold Decision
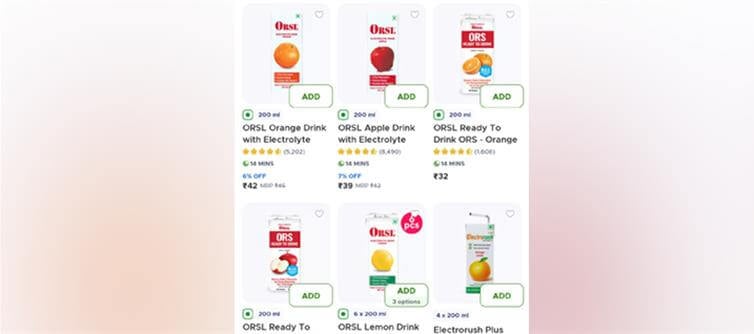
For nearly a decade, indian households have been tricked into believing that bottled 'ORS' drinks sold in supermarkets and quick-commerce apps like Blinkit and Zepto were medically approved oral rehydration solutions. In reality, most of these products were nothing more than sugary electrolyte beverages dressed in the garb of life-saving formulations. On October 14, 2025, the Food Safety and Standards Authority of india (FSSAI) finally woke up — banning the use of 'ORS' on any product that doesn't comply with WHO-approved medical composition standards.
It’s a long-overdue strike against corporate deception and regulatory lethargy.
The 8-Year Delay: When 'Health' Became a Marketing Gimmick
Since 2017, several beverage brands have cleverly exploited a loophole — using the term 'ORS' (Oral Rehydration Solution) to market flavored, non-medical drinks. These products often contained excess sugars, artificial flavors, and incorrect electrolyte ratios, making them medically useless and sometimes harmful for dehydration patients.
For eight long years, FSSAI issued warnings but failed to enforce a clear ban. Companies laughed their way to the bank while parents unknowingly gave their children these pseudo-health drinks during fevers, heat strokes, and diarrheal illnesses.
This wasn’t just misleading advertising — it was corporate malpractice disguised as nutrition.
The FSSAI Ban: Finally, a Reality Check
On October 14, 2025, FSSAI officially banned the use of the term 'ORS' on all non-WHO-compliant beverages. The move came after repeated pressure from medical associations and consumer watchdogs who accused brands of creating dangerous confusion between therapeutic ORS (a WHO-formulated rehydration solution) and flavored drinks sold under misleading labels.
The order explicitly prohibits any company from using 'ORS' or 'Oral Rehydration Solution' unless their product meets the WHO/ICMR electrolyte and glucose standards. Any violation now falls under misbranding and deceptive advertising — a punishable offense under India’s food safety laws.
The Legal Twist: court Grants Stay to J&J’s ORSL
But just when consumers thought justice had prevailed, the story took a sharp legal turn. On October 17, 2025, the Delhi High court granted interim relief to Johnson & Johnson, allowing it to sell existing stocks of its flagship drink ORSL, worth approximately ₹155-180 crore.
This means that despite the FSSAI ban, some of these so-called 'ORS' beverages may still appear on e-commerce apps like Blinkit, swiggy Instamart, and amazon Pantry — legally, at least until the existing inventory runs out.
So yes, you may still find banned-in-spirit-but-legal-in-stock products online for now. But make no mistake: their time is up.
The Accountability Void: Why Blinkit and Others Must Act Fast
Even if certain products are temporarily protected by a court stay, platforms have a moral responsibility to protect consumers. Blinkit, Zepto, amazon, and flipkart cannot hide behind technicalities. Selling products under misleading labels directly impacts public trust.
These companies must act decisively — by flagging such listings, issuing disclaimers, or voluntarily removing them until legal clarity is achieved. Otherwise, they risk becoming accessories to deception in the eyes of consumers.
Democracy at Half Speed: When Truth Takes a Decade to Arrive
The FSSAI’s ban is a step in the right direction, but it also exposes a painful truth — India’s regulatory system often wakes up only after years of damage. It took eight years, hundreds of complaints, and health experts shouting themselves hoarse before something as basic as label honesty was enforced.
This is not just a food safety issue. It’s a mirror to India’s deeper governance problem — where truth and accountability move more slowly than profit and propaganda.
Bottom Line: Ban Is Welcome, But Justice Is Late
FSSAI’s crackdown on fake ORS products is a victory for consumer health, but a bittersweet one. The damage has already been done. Millions were misled, thousands of children consumed unverified drinks, and brands built empires on false credibility.
The ban must now be followed by strict market clean-up, prosecution, and recall drives — not just paperwork and court stays.




 click and follow Indiaherald WhatsApp channel
click and follow Indiaherald WhatsApp channel